
Some molecules do not have any atoms attached to four different groups but are still chiral as a result of the molecular architecture. The chirality for complex molecules can only be determined absolutely by creating mirror images of the original molecule and checking their superimposition. The most certain way of finding out enantiomers is to draw mirror images and check their superimposition.Īnother point to note is that the presence of an atom with four different groups attached to it is not in itself sufficient for the molecule to be chiral.
#STERIOISOMERS THAT DIFFER AT ANOMERIC CARBON HOW TO#
This puts forth the question, how to find stereoisomers for a molecule. In other words, a meso compound is a stereoisomer of a chiral compound that has a superimposable mirror image. This molecule can be termed as a meso compound that has a stereogenic atom, even though it is achiral. This results in the formation of three isomers- two enantiomers and one achiral molecule. This happens due to the plane of symmetry that is present within the compound. This happens in compounds with high symmetry.įor e.g., 2,3-dichlorobutane does not have SS and RR enantiomers but an identical pair of SR isomers. The 2 n number of stereoisomers formula fails to provide the real number of stereoisomers in some cases. Therefore, the 2 n rule states that a molecule with ‘n’ number of chiral atoms present may have 2 n stereoisomers. The number of stereoisomers increases exponentially with an increase in the number of stereocenters. Number of Stereoisomers= 2 n, where n represents the number of stereogenic centres present in the molecule. This can be generalized into the stereoisomers formula, which is: Similarly, in the case of three chiral atoms, there are eight possible combinations for stereoisomers: RRR, RRS, RSR, SRR, SSR, SRS, RSS, and SSS. This leads to four possible stereoisomers: RR, SS, RS and SR. Taking this into account, the answer to how to find number of stereoisomers can be found.įor a molecule with two stereocenters, both can be either R or S. A molecule with one stereocenter can have two stereoisomers. With an increase in the complexity of molecules, the number of stereocenters also increases. Now, what happens as the molecules become more complex? Thus, every stereocenter can have two stereoisomers. This carbon atom with four different groups attached to it is termed as the chiral centre or stereocenter of the molecule.Ĭhanging the positions of the substituent can result in two possible arrangements, denoted by R and S. These substituents are arranged in such a way that prevents superimposition of the mirror images, even though they have the same substituents attached. Such molecules have one or more carbon atoms with four nonidentical substituents. the mirror images are distinguishable), they are termed chiral.
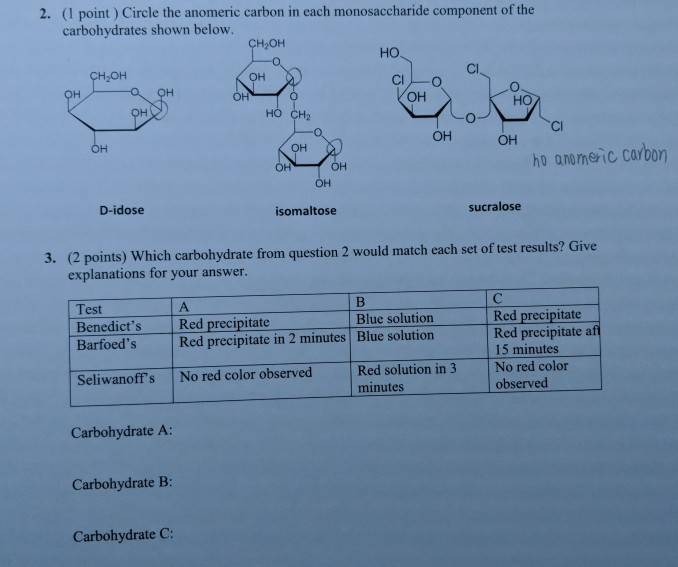
When the mirror images of two isomers are not superimposable (i.e. They have different R and S configurations.ĭiastereomers have the same bond configuration at one stereocenter and differ in configuration at other centres. Two or more stereogenic centres are present. They have one or more stereogenic centres. These have varying physical and chemical properties. Stereoisomers that do not form mirror images of each other are termed as diastereomers.Īll have the same physical and chemical properties (except interaction with light). Stereoisomers that are non-superimposable images of each other. Diastereomers, on the other hand, are stereoisomers that do not form mirror images.ĭifference Between Enantiomers and Diastereomers These can be understood by one’s hands which are mirror images of each other. Stereoisomers that form mirror images of each other are termed as enantiomers. These isomers can be classified into two types- enantiomers and diastereomers.īefore we find out how to calculate stereoisomers, it is important to learn the types of stereoisomers and the distinctions between them. Isomers that comprise the same parts but differ in spatial orientation are termed as stereoisomers. (ii) Stereoisomers- On the contrary, the stereoisomer of a molecule will have the same connectivity but differ in orientation in space. They can be further classified into chain, position and functional groups isomers. (i) Constitutional Isomers- These isomers have the same parts but differ in connectivity.
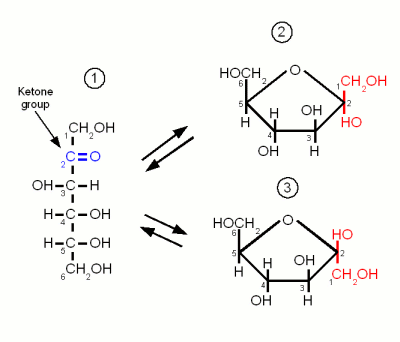
Isomers can be generally classified into two types. These compounds also differ in their physical and chemical properties. In simple terms, they have the same constituents but differ in structure and characteristics. Isomerism refers to the existence of compounds that have the same formula but different structure.


 0 kommentar(er)
0 kommentar(er)
